Research Interests
1. Cellular and molecular mechanisms underlying plasma sodium detection by the brain and their role in the salt-sensitive hypertension. Specifically, we study a function of specialized osmosensory neurons that are activated by increased levels of sodium in the blood. These neurons release antidiuretic hormone vasopressin to stabilize the levels of sodium and water in the circulation. We investigate the role of unique cytoskeletal structures and signalling molecules featured by these neurons, and study how these elements are affected by high dietary salt, mediating hyperactivation of osmosensory neurons, and thereby contributing to hypertension.
2. Cellular and molecular mechanisms underlying the communication between the brain and the peripheral circulation: the role of non-neuronal cells (tanycytes, astrocytes, pericytes, and endothelial cells) in the regulation of the blood brain barrier. Metabolites, hormones and other circulating molecules cannot freely penetrate into the brain due to the existence of the blood brain barrier, which isolates the brain from molecules found in the peripheral circulation. Specialized small brain regions in the hypothalamus (called circumventricular organs) are lacking the complete blood brain barrier. Therefore, blood-borne circulating molecules can partially access these areas to influence the activity of local neurons, which in turn generate neuronal responses by mediating hormonal release, promoting behaviors (e.g. by triggering thirst or hunger), or activating autonomic nerve system to modulate cardiovascular system and renal function. Our goal is to understand the cellular and molecular mechanisms that regulate the access of peripheral signals to neurons in the circumventricular organs and to study functions of non-neuronal cells (tanycytes, astrocytes, pericytes, and endothelial cells) in this dialogue between the periphery and the central nervous system.
3. Cellular and molecular mechanisms underlying the regulation of the blood brain barrier in health and metabolic disorders. Tanycytes have been proposed to serve as gatekeepers mediating the communication between the brain and the body at hypothalamic circumventricular organs. Tanycytes can adjust by altering the functional and structural organization of the blood-hypothalamus barrier in response to nutritional status of an individual (e.g. fasting vs feeding). We investigate molecular and cellular mechanisms that underlie these plastic changes, and study how disruption of these processes leads to metabolic disorders promoting obesity, diabetes, and hypertension.
To address these questions, we use a variety of techniques:
- Patch clamp electrophysiological recordings (brain slices, dissociated cells)
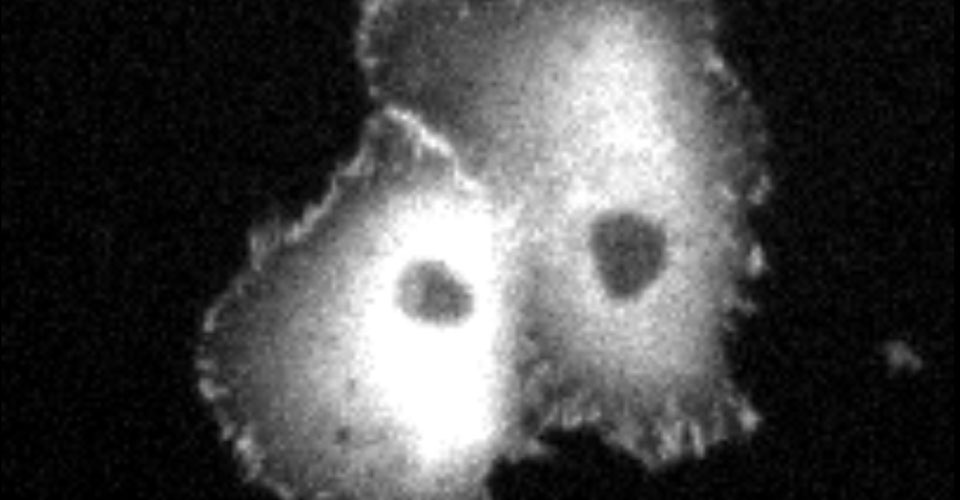
- Super-resolution imaging
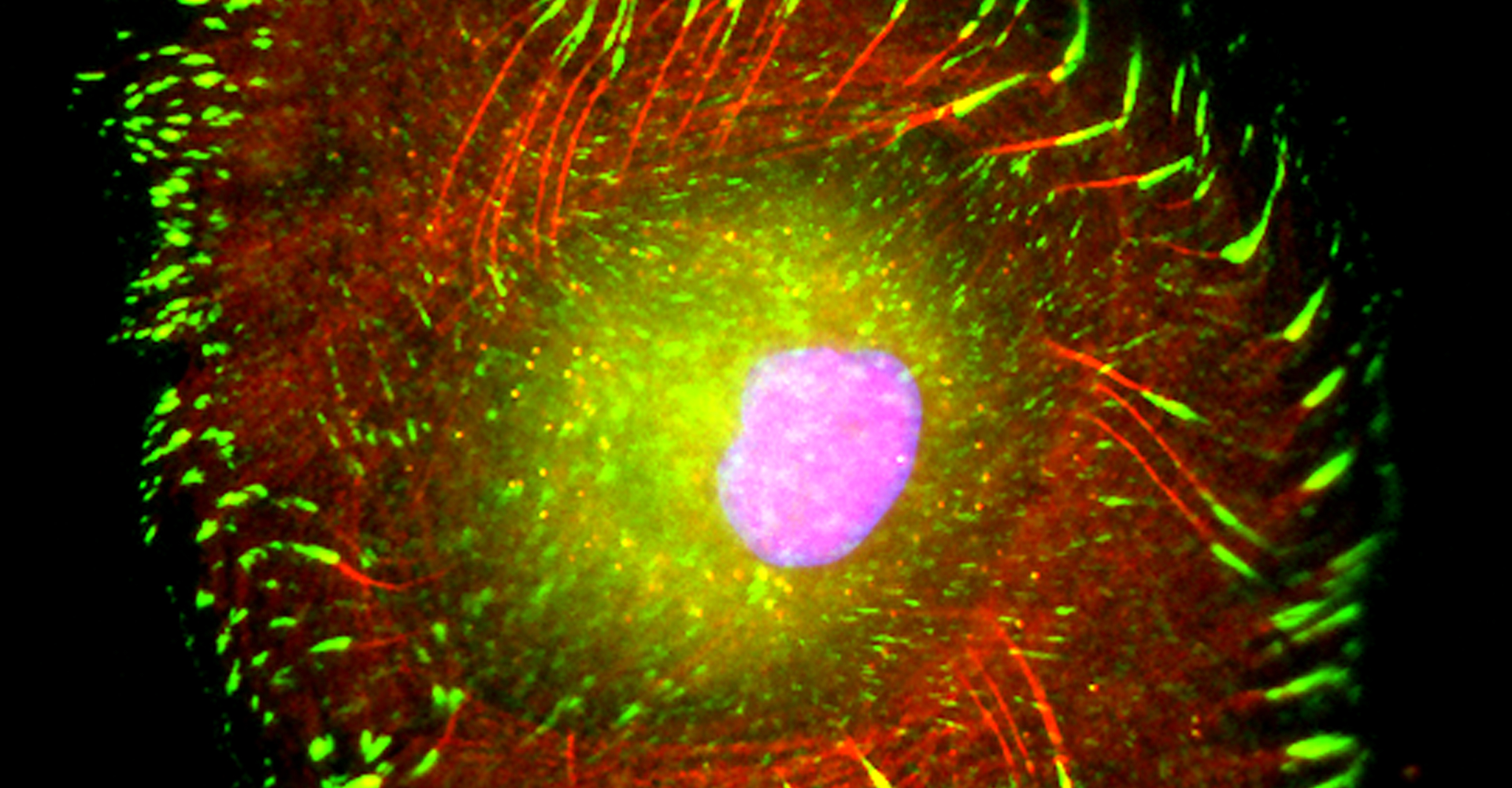
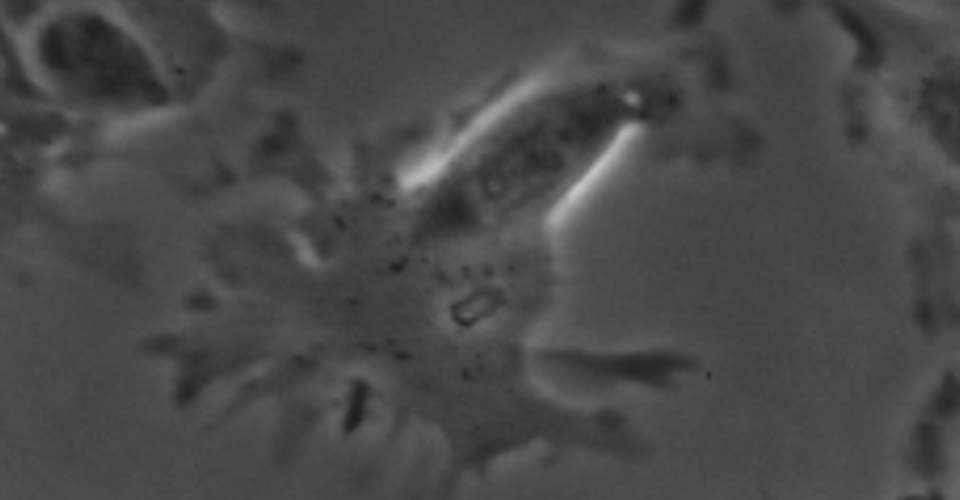
- Live-cell imaging (calcium, cytoskeleton, and signaling molecules)
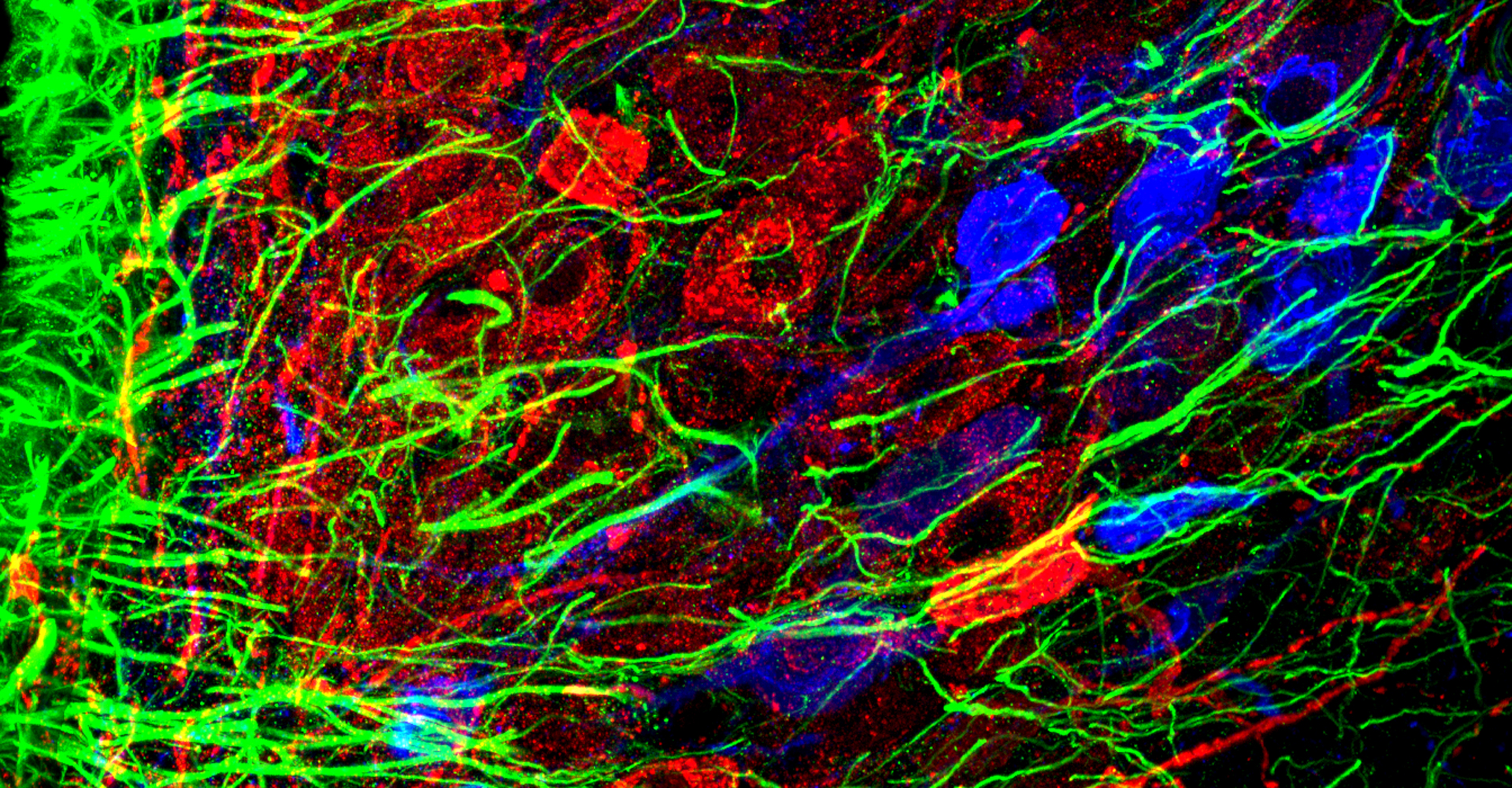
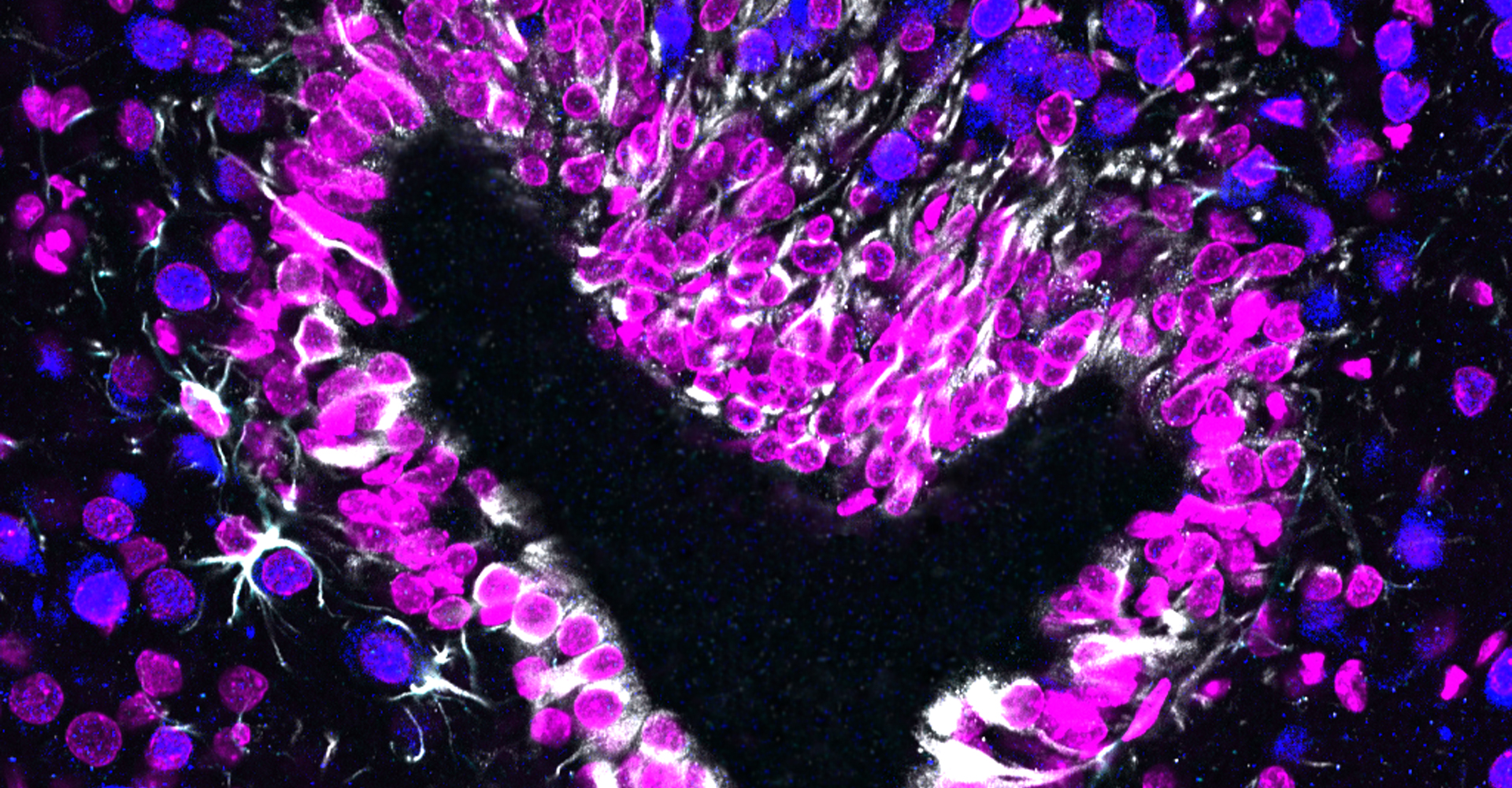
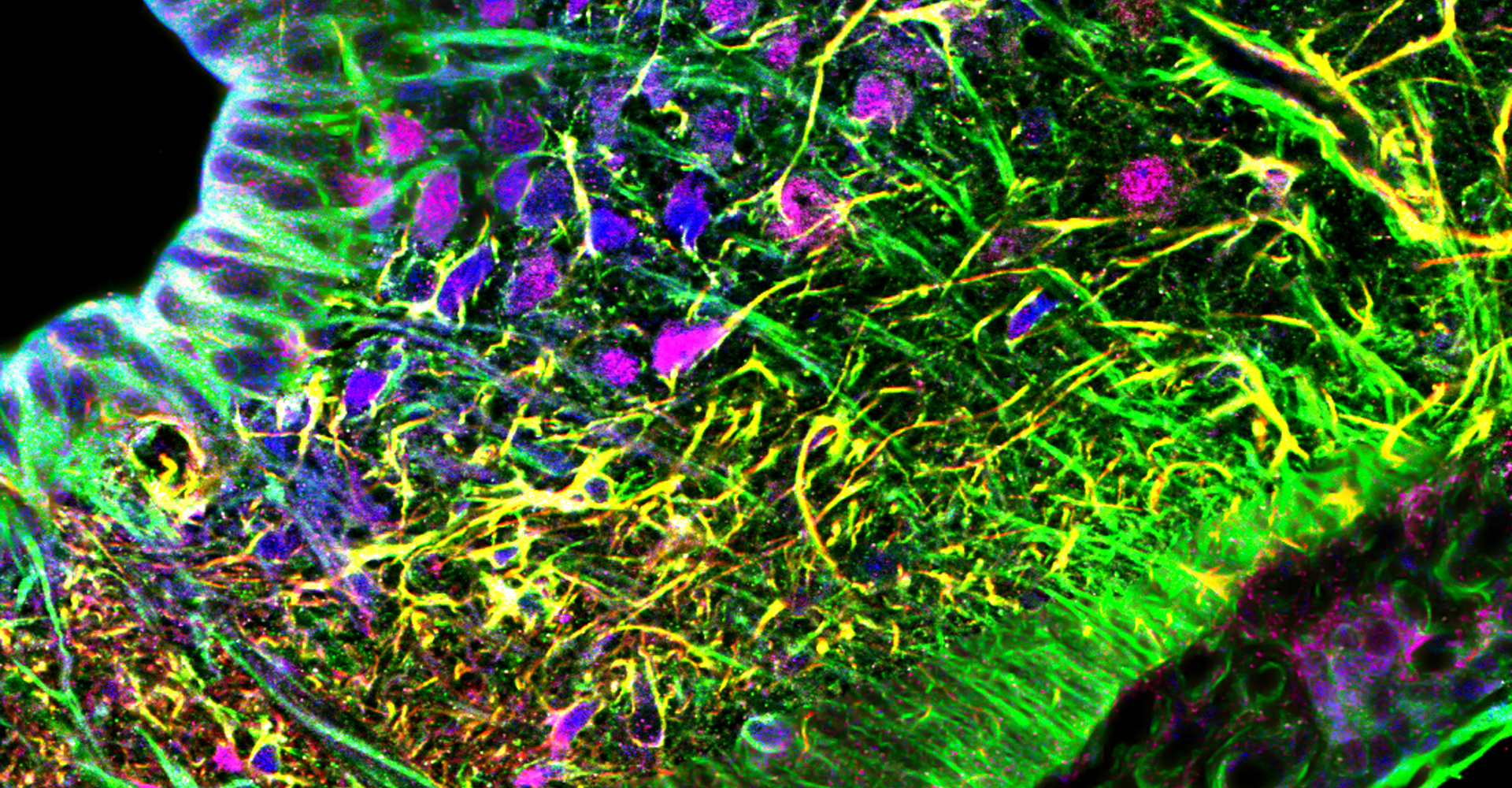
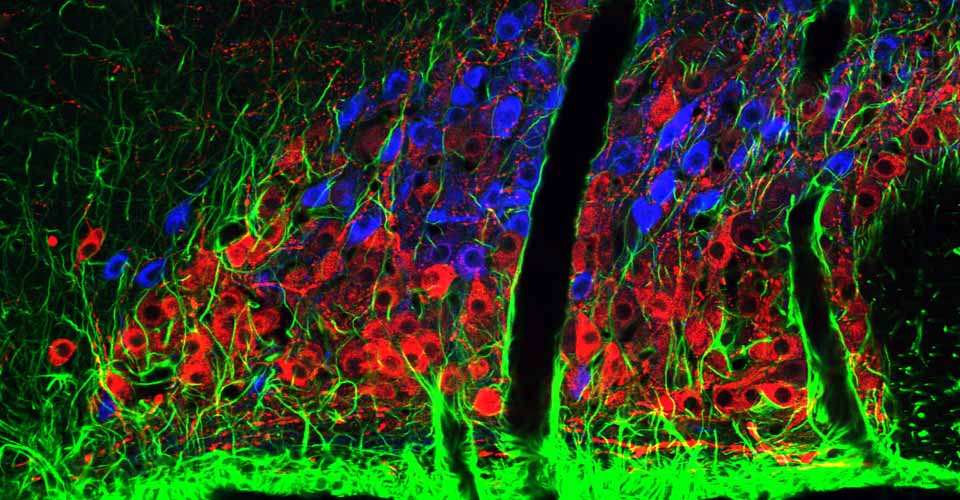
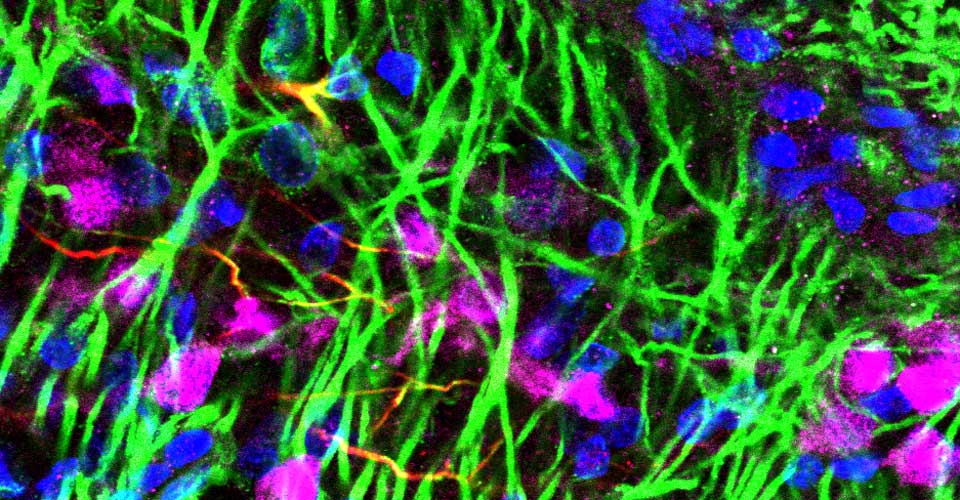
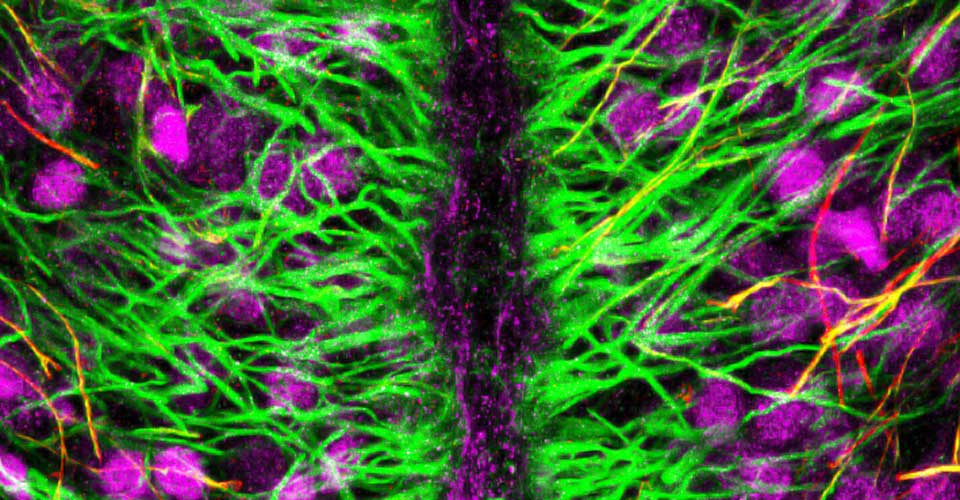
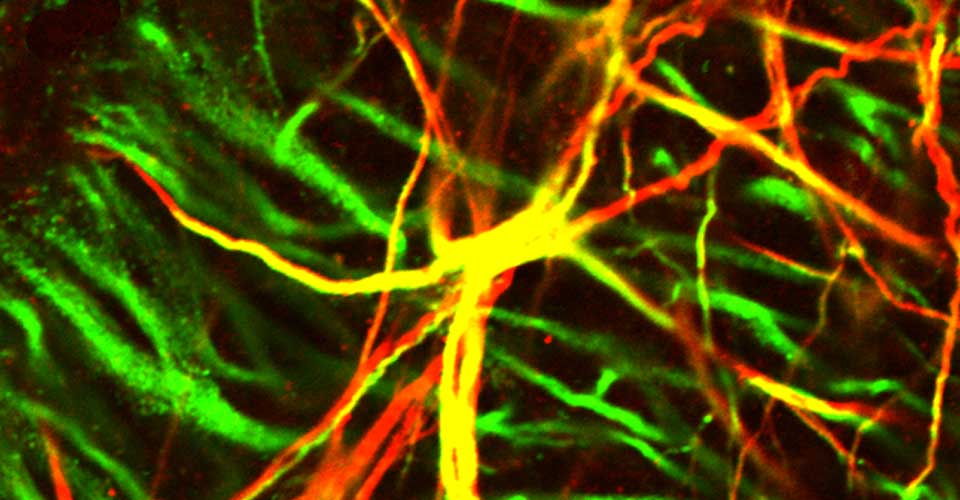
- Immunohistochemistry, histology, and neuronal-glial-vasculature morphometry
- Hemodynamic measurements (blood pressure and heart rate)
- Animal models of human diseases

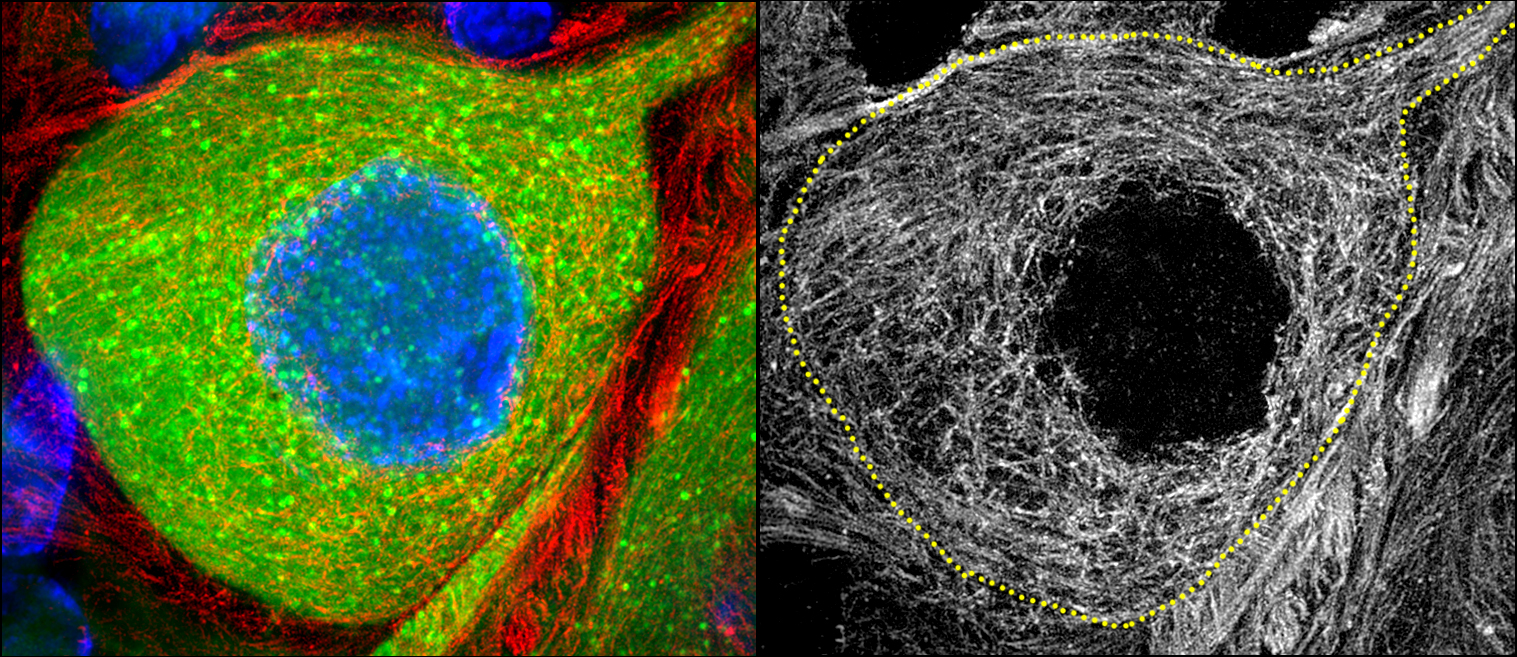
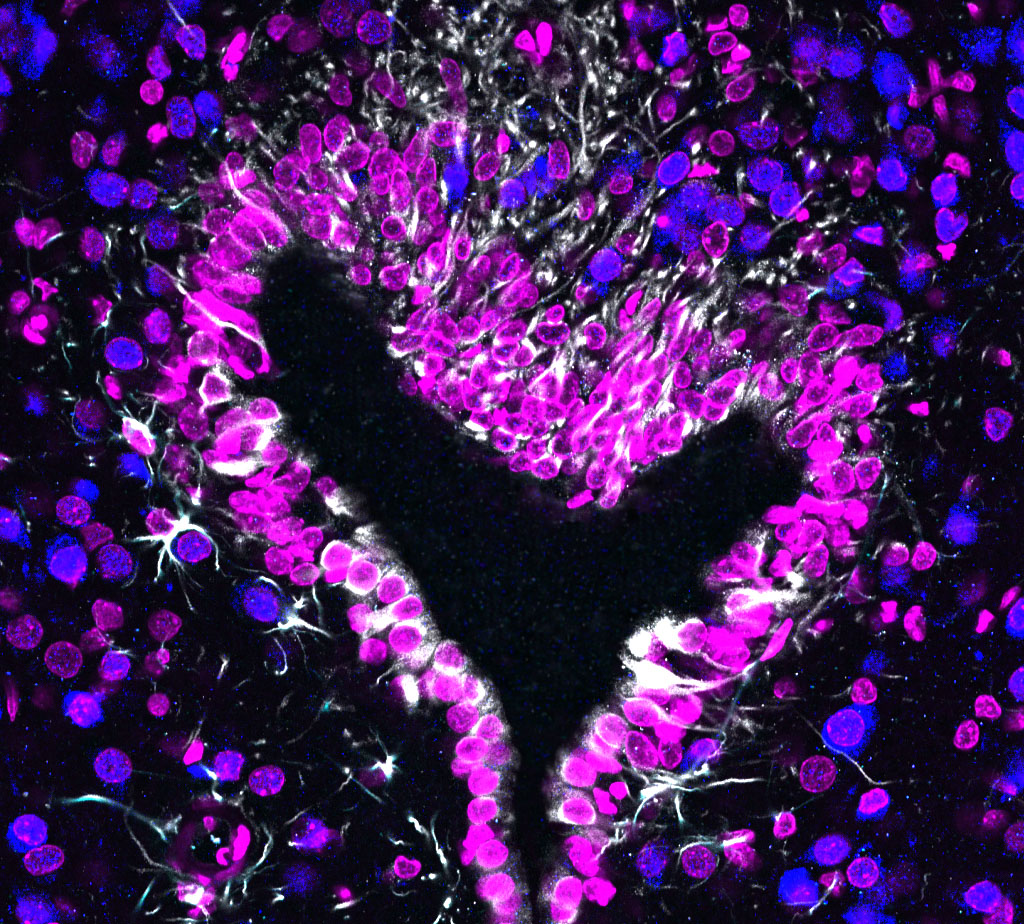
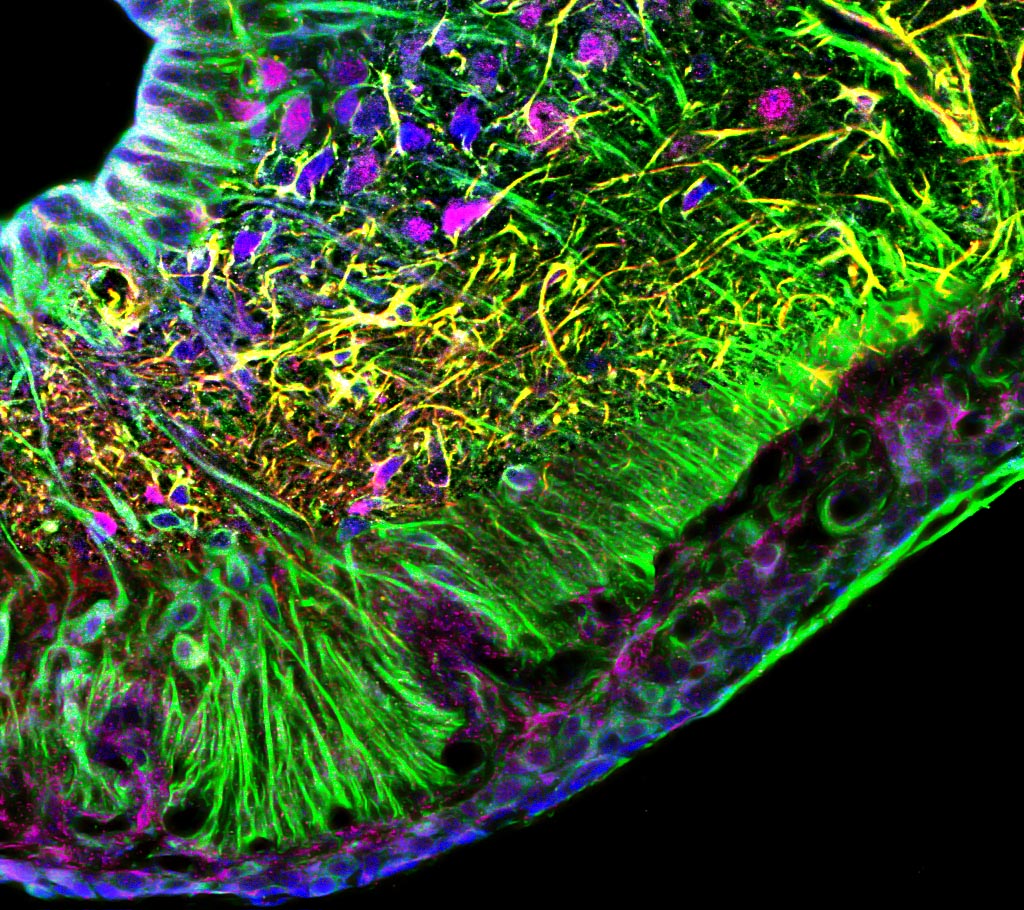
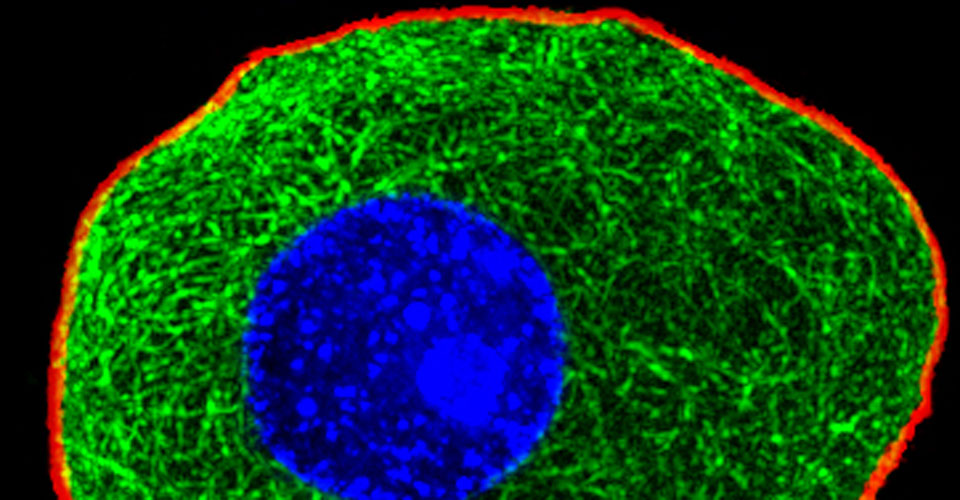
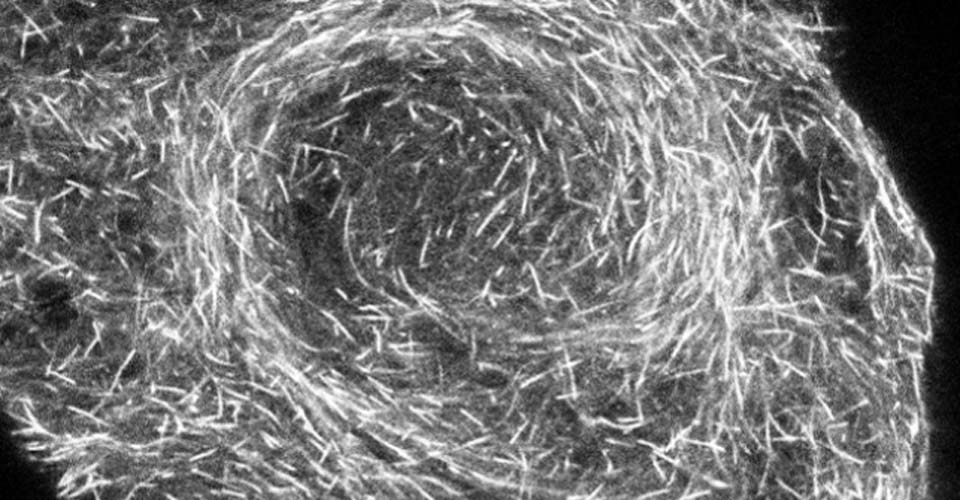
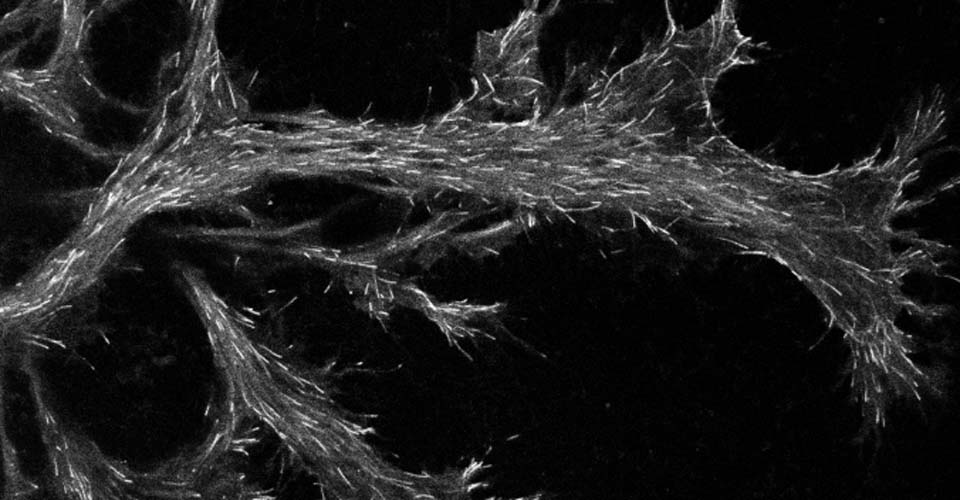
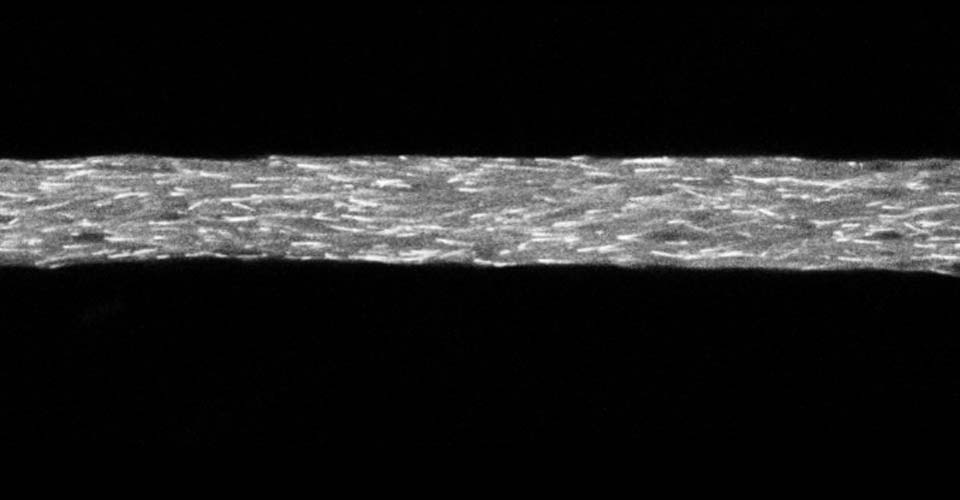
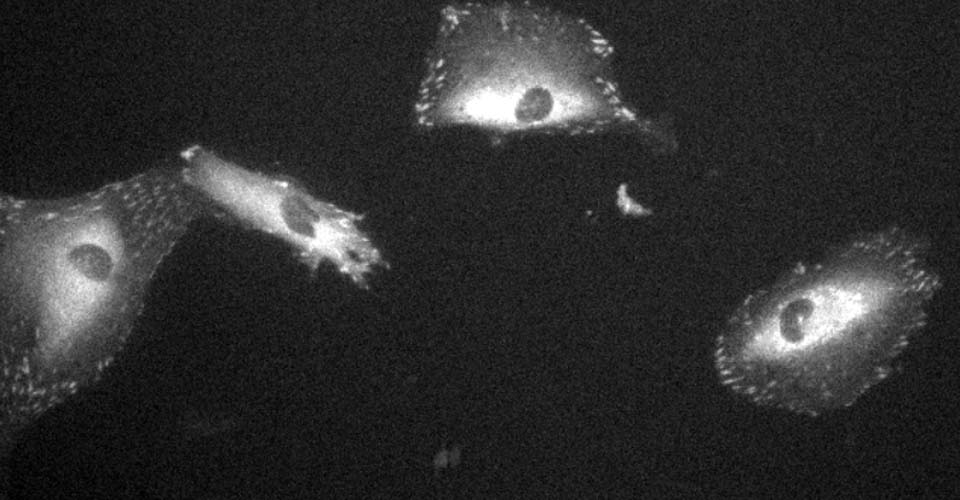








 I received my PhD from the Hebrew University of Jerusalem, Israel, where I investigated the interplay between cytoskeleton and plasma membrane dynamics (endo- and exocytosis) under normal conditions and during axonal regeneration. I characterized the important role of crosstalk between membrane recycling and cytoskeleton dynamics in regulation of neuronal morphology.
I received my PhD from the Hebrew University of Jerusalem, Israel, where I investigated the interplay between cytoskeleton and plasma membrane dynamics (endo- and exocytosis) under normal conditions and during axonal regeneration. I characterized the important role of crosstalk between membrane recycling and cytoskeleton dynamics in regulation of neuronal morphology.







